TIP4P/2005 model of water: Difference between revisions
Jump to navigation
Jump to search
m (Slight tidy.) |
m (Slight tidy.) |
||
| Line 2: | Line 2: | ||
<ref>[http://dx.doi.org/10.1063/1.2121687 J. L. F. Abascal and C. Vega "A general purpose model for the condensed phases of water: TIP4P/2005", Journal of Chemical Physics, '''123''' 234505 (2005)]</ref> | <ref>[http://dx.doi.org/10.1063/1.2121687 J. L. F. Abascal and C. Vega "A general purpose model for the condensed phases of water: TIP4P/2005", Journal of Chemical Physics, '''123''' 234505 (2005)]</ref> | ||
is a re-parameterisation of the original [[TIP4P]] potential for [[Computer simulation techniques | simulations]] of [[water]]. | is a re-parameterisation of the original [[TIP4P]] potential for [[Computer simulation techniques | simulations]] of [[water]]. | ||
TIP4P/2005 is a rigid planar model, having a similar geometry to the Bernal and Fowler | TIP4P/2005 is a rigid planar model, having a similar geometry to the [[BF |Bernal and Fowler model]]. | ||
==Parameters== | ==Parameters== | ||
[[Image:Water_empirical1.png|center|300px]] | [[Image:Water_empirical1.png|center|300px]] | ||
Revision as of 15:00, 19 May 2009
The TIP4P/2005 model [1] is a re-parameterisation of the original TIP4P potential for simulations of water. TIP4P/2005 is a rigid planar model, having a similar geometry to the Bernal and Fowler model.
Parameters
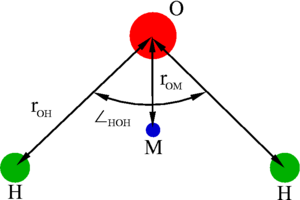
| (Å) | HOH , deg | (Å) | (K) | q(O) (e) | q(H) (e) | q(M) (e) | (Å) |
| 0.9572 | 104.52 | 3.1589 | 93.2 | 0 | 0.5564 | -2q(H) | 0.1546 |
Phase diagram
The phase diagram of the TIP4P/2005 model is given in a publication by Abascal, Sanz and Vega.
Liquid-vapour equilibria
Plastic crystal phases
Recent simulations have suggested the possibility of a plastic crystal phase or phases for water.
Surface tension
The surface tension has been studied for the TIP4P/2005 model by Vega and Miguel.
Self-diffusion coefficient
The TIP4P/2005 potential has a self-diffusion coefficient, in bulk water at 298 K, of 0.21 Å2 ps−1 in a classical simulation of 216 water molecules (experimental value: 0.23 Å2 ps−1).




